Cancer metastasis is one of the most important unsolved problems in human health.
Some 90% of cancer deaths are the result of metastasis, a process in which cells leave a primary tumour and seed secondary tumours. These secondary tumours can be substantial in number, can bewidely disseminated and can occur in critical organs such as the brain. These factors make them difficult, if not impossible, to remove surgically, and they are the reason that metastasis represents such a serious health condition.
We argue that metastasis is fundamentally a mechanics problem, because its defining feature is the movement of cells, as described in this short video (Video 1).
Video 1 - Could cancer metastasis be prevented?
If the movement of cancer cells away from a primary tumour could be arrested, metastasis could be prevented.
As a next step, we needed to figure out the nature of the forces that drive metastatic cell movements. These cell movements are very difficult to observe directlysince they occur internally, and compared to the number of cells present, they are quite rare. Not only that, but techniques to measure cellular forces are very much in their infancy.
So, we decided to use a computational model – a virtual reality that is built on the laws of physics(rather than a game-builder’s imagination) to figure out these forces. To that end, we adapted a model that we had developed to learn about the forces that drive cell motions during early embryo development. The model had been carefully tested over several decades, and it had enabled us to learn much about the forces that cause single cells and groups of cells to move as an embryo is formed [see References 1-4].
We used our computational model to investigate a number of the steps through which metastasis occurs, as shown in Figure 1 [5].Our model helped us to learn about the particular combinations of forces that must act at various steps in this process [5], and it revealed the forces needed for a cell to break away from a primary tumour (Step B), the initial step in metastasis.
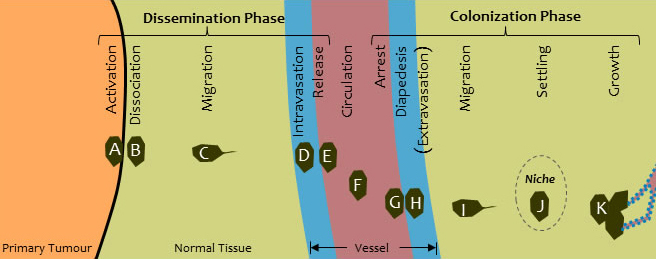
Figure 1 - The steps in metastasis (from reference [5])
Using the model, we were able to figure out specific minimum combinations of forces that a single metastatic cell (the dark cell) must generate in order to break away from a primary tumour (shown in orange), migrate through healthy tissue (green), invade the wall of a blood or lymph vessel (blue) and be released into the blood or lymph flow (red). The computer simulation below (Video 2) illustrates these steps.
VIdeo 2 - A computer simulation (model) of the steps in the dissemination phase of metastasis [5].
Special attention was paid to the forces needed for a single cell to break away from a primary tumour, as shown in this animation (Video 3).
Video 3 - The process of dissociation [5]
Our study revealed specific mechanical conditions, in terms of interfacial tension, that must be satisfied for a cancer cell to escape a primary tumour (Figure 2), and it suggested some biological pathways that might be useful for manipulating these forces and interfering with cancer cell escape.
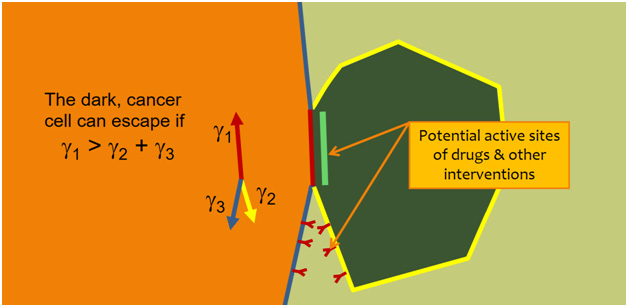
Figure 2 - One of the conditions that must be satisfied for a cancer cell to escape a primary tumour [5]
A new force inference technique we developed [6,7] has proved effective in assessing the motility of murine breast duct cells from their shapes [8], and it may prove effective in assessing the metastatic potential of cancer cells and the mechanical effectiveness of potential treatment strategies.
Again, if the motions of cancer cells could be arrested, cancer metastasis could finally be prevented, and many lives saved.
References
- Brodland, G.W. and Chen, H.H., 2000a, "The Mechanics of Heterotypic Cell Aggregates: Insights from Computer Simulations", ASME Journal of Biomechanical Eng., Vol. 122, pp. 402-407.
- Brodland, G.W., 2002, "The Differential Interfacial Tension hypothesis (DITH): A Comprehensive Theory for Self-Rearrangement of Embryonic Cells and Tissues," ASME Journal of Biomechanical Engineering, Vol. 124, pp. 188-197.
- Brodland, G.W., Chen, X., Lee, P. and Marsden, M., 2010, "From genes to neural tube defects (NTDs): Insights from multiscale computational modeling," HFSP Journal, Vol. 4, pp. 142-152. doi: 10.2976/1.3338713.
- ] SF Gabriel Krens, Jim H Veldhuis, Vanessa Barone, Daniel Čapek, Jean-Léon Maître, G Wayne Brodland, Carl-Philipp Heisenberg, 2017, Interstitial fluid osmolarity modulates the action of differential tissue surface tension in progenitor cell segregation during gastrulation, Development, 144, 1798-1806. doi: 10.1242/dev.144964
- Brodland, G.W. and Veldhuis, J.H., 2012, "The Mechanics of Metastasis: Insights from a Computational Model," PLoS ONE, Vol 7, No. 9, e44281, pp. 1-11. doi:10.1371/journal.pone.0044281.
- Brodland, G.W, Veldhuis, J.H., Kim, S., Perrone, M., Mashburn, D., Hutson, M.S., 2014, "CellFIT: A Cellular Force-Inference Toolkit Using Curvilinear Cell Boundaries," PLoS ONE, Vol. 9, No. 6, e99116. doi: 10.1371/journal.pone.0099116.
- Jim H Veldhuis, Ahmad Ehsandar, Jean-Léon Maître, Takashi Hiiragi, Simon Cox, G Wayne Brodland, 2017, "Inferring cellular forces from image stacks”, Philosophical Transactions of the Royal Society B, 372, 20160261. doi: 10.1098/rstb.2016.0261.
- Neil M Neumann, Matthew C Perrone, Jim H Veldhuis, Robert J Huebner, Huiwang Zhan, Peter N Devreotes, G Wayne Brodland, Andrew J Ewald, 2018, “Coordination of Receptor Tyrosine Kinase Signaling and Interfacial Tension Dynamics Drives Radial Intercalation and Tube Elongation”,Developmental Cell, 45, 67-82, e6 doi.org/10.1016/j.devcel.2018.03.011